Pain

Welcome
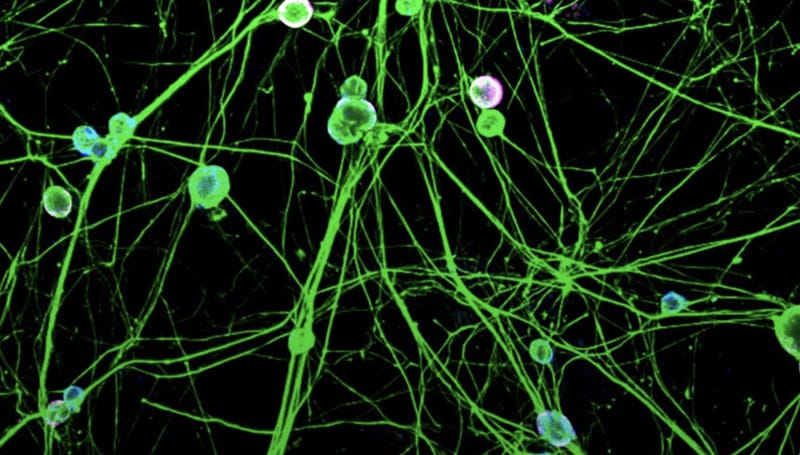
Chronic pain affects millions of people worldwide and is a leading cause of disability. Its prevalence is greater than heart disease, cancer, and diabetes combined, yet, safe, effective and non-addictive treatments are lacking. Opiates are currently used to treat chronic pain despite their relative ineffectiveness and high potential for misuse and addiction. Better treatments are urgently needed. Studies of human pain disorders and experimental models of pain have validated Nav1.7, a voltage-gated sodium channel, as a highly suitable target for the development of new, non-addictive pain treatments. Patients with overactive Nav1.7 exhibit severe pain syndromes, and those with defunct Nav1.7 feel little to no pain and no apparent cardiac or cognitive deficits.
Although blockers of Nav1.7 such as local anesthetics and anticonvulsants can provide some symptomatic relief, they exert worrisome side effects on the heart and brain. This is because these blockers are non-selective, in that they block not only Nav1.7 but also other types of sodium channels that are required for normal functioning of the heart and central nervous system. The development of selective Nav1.7 blockers and genetic approaches such as knockdown or suppression of expression as a novel analgesic is currently an area of active investigation. Targeting of Nav1.7 would be expected to carry fewer side effects compared to current analgesics. Edit Chronic Pain (ECP) is a research consortium, funded under the title, “Site Directed RNA Editing as a Novel Analgesic,” by the NIH HEAL Initiative: Team Research for Initial Translational Efforts in Non-addictive Analgesic Therapeutics Development. The mission of the consortium is to develop and validate an innovative strategy in which Nav1.7 is targeted in a highly selective manner via site-directed RNA editing to alleviate chronic pain.
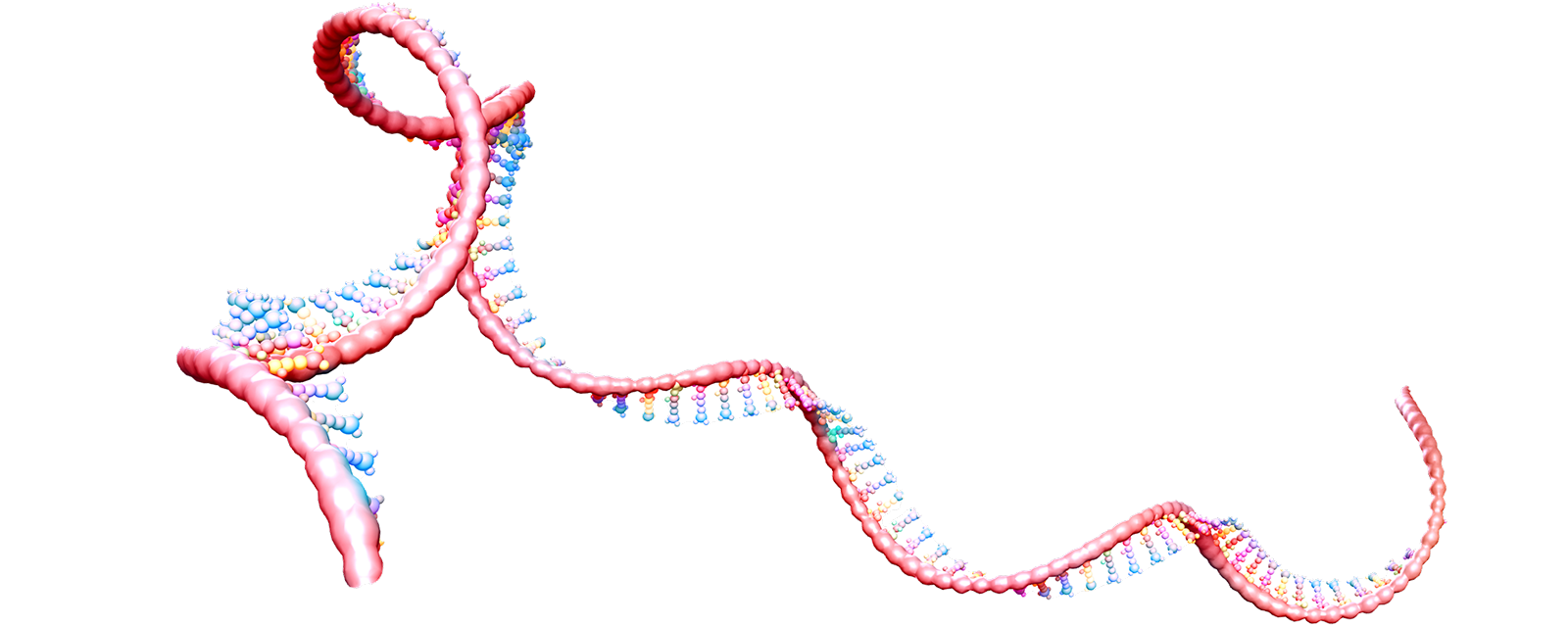
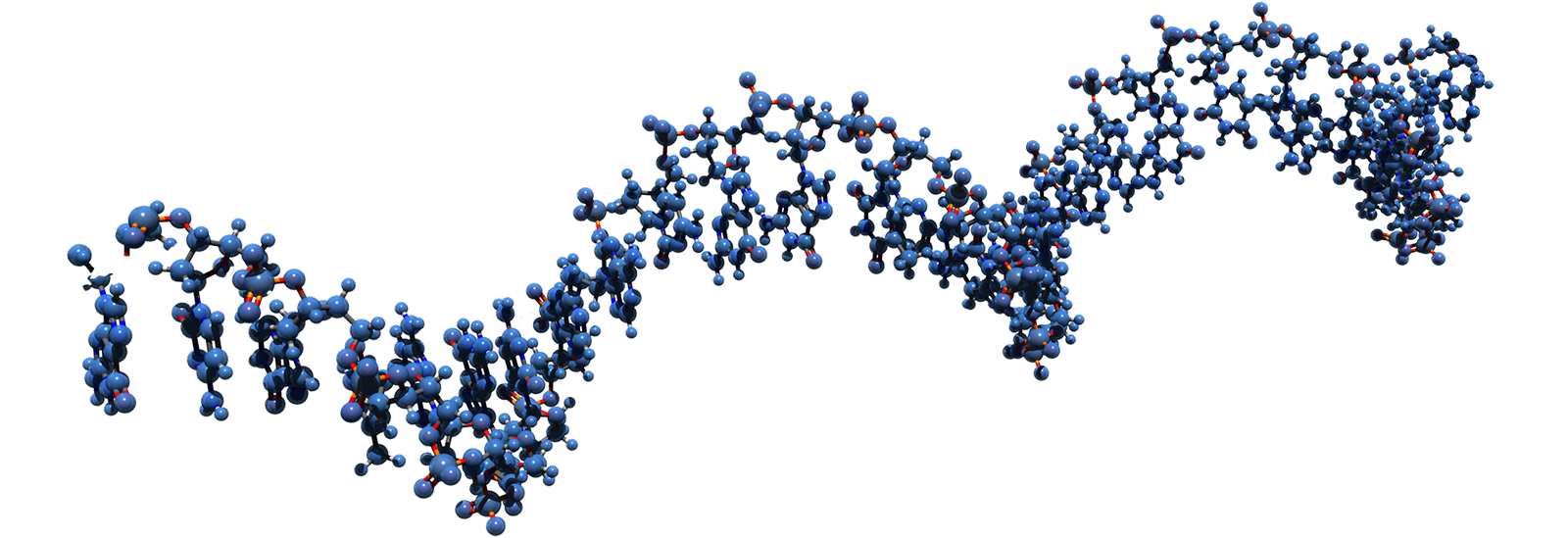
Site-directed RNA editing consists of intercepting and editing the RNA message of the Nav1.7 gene before the channel is made. The editing results in a Nav1.7 channel with a selectivity filter that is permeable to sodium and potassium ions but not calcium ions, and thus act as an electric shunt that attenuates firing of neurons. This approach to analgesia has the additional advantage of being delivered in a regional and tissue-specific manner, which reduces the potential for side effects. Another advantage of this approach is that unlike gene editing, for example using CRISPR, which alter the genome permanently and may lead to unintended consequences, RNA editing is transient and normal channel function is restored when delivery of editing reagents is suspended and the edited channel protein is turned over.
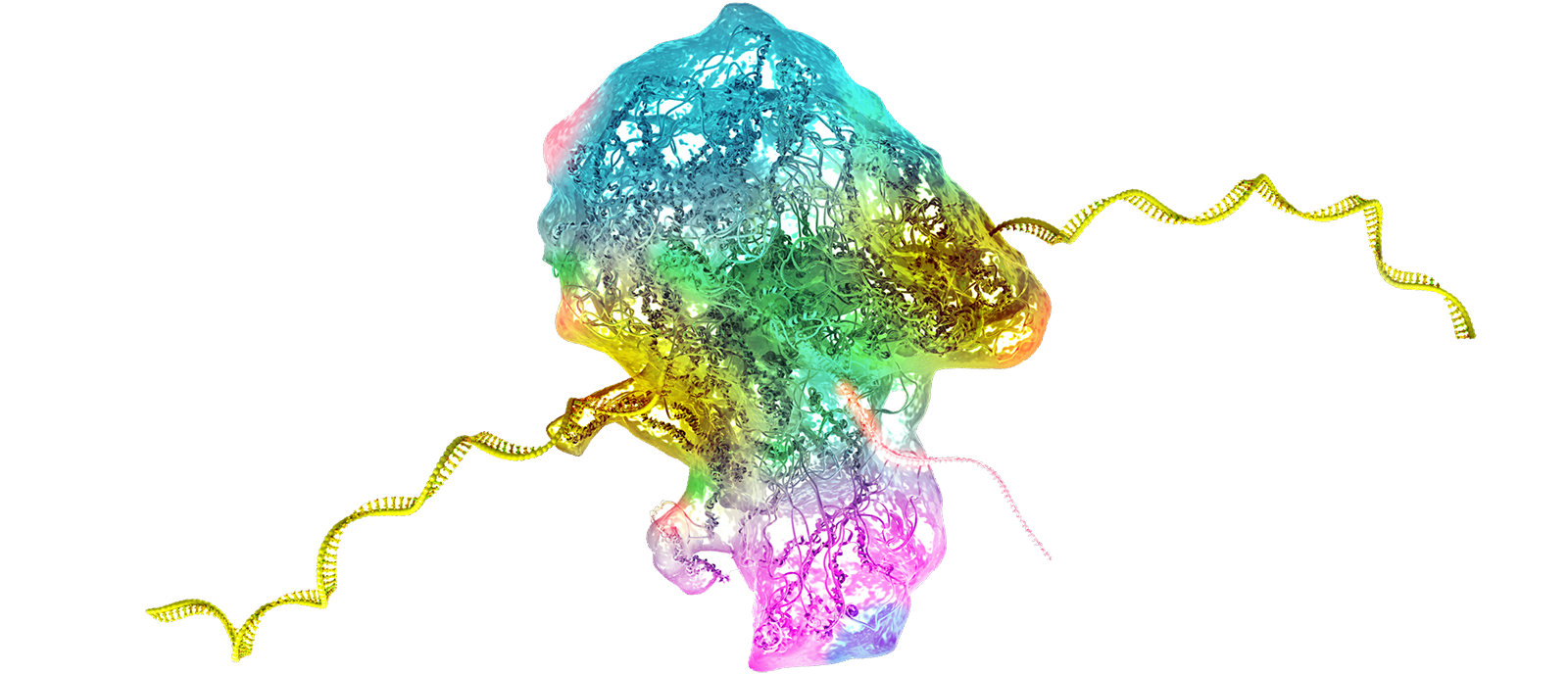
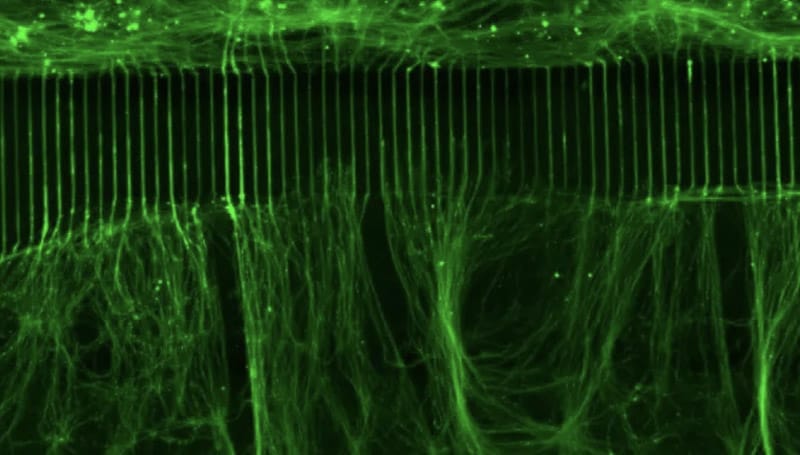
The consortium includes a multidisciplinary team of experts in molecular neuroscience, neurobiology of pain and its co-morbidities, pharmacology, physiology, and bioinformatics. The participating institutions are: The Marine Biological Laboratory, Yale University, University of Texas at Dallas, and Tel Aviv University. Together they will work to apply RNA editing technology to modify Nav1.7 in a manner that calms overactive pain-signaling nerves in pre-clinical experimental models of chronic pain, and in cell-based assays using human sensory neurons, which provide preclinical validation toward translation to human clinical trials.
Participating Institutions
Key Publications
RNA editing as a strategy to transiently alter protein function
Reardon, S. Step aside CRISPR, RNA editing is taking off. Nature. News Feature. 4 February 2020.
Vallecillo-Viejo IC, Liscovitch-Brauer N, Diaz Quiroz JF, Montiel-Gonzalez MF, Nemes SE, Rangan KJ, Levinson SR, Eisenberg E, Rosenthal JJC. Spatially regulated editing of genetic information within a neuron. Nucleic Acids Res. 2020 May 7;48(8):3999-4012. doi: 10.1093/nar/gkaa172. PMID: 32201888; PMCID: PMC7192619.
Liscovitch-Brauer, N., Alon, S., Porath, H.T., Elstein, B., Unger, R., Ziv, T., Admon, A., Levanon, E.Y., Rosenthal, J.J.C.*, and Eisenberg, E.* (2017). Trade-off between transcriptome plasticity and genome evolution in cephalopods. Cell. 169:191-202. DOI: 10.1016/j.cell.2017.03.025. PMCID: PMC5499236.
M.F. Montiel-Gonzalez, I. Vallecillo, G. Yudowski, and J.J.C. Rosenthal (2013). Correction of mutations within the cystic fibrosis transmembrane conductance regulator by site-directed RNA editing. PNAS 110: 18285-90. DOI: 10.1073/pnas.1306243110. PMCID: PMC3831439.
M.F. Montiel-Gonzalez, I.C. Vallecillo-Viejo, and J.J.C. Rosenthal (2016). An efficient system for selectively altering genetic information in mRNAs. Nucl. Acids. Res. 44: e157. DOI: 10.1093/nar/gkw738. PMCID: PMC5137428.
Roth, SH, Levanon, EY, Eisenberg, E. (2019). Genome-wide quantification of ADAR adenosine-toinosine RNA editing activity. Nature Methods. 16:1131-1138.
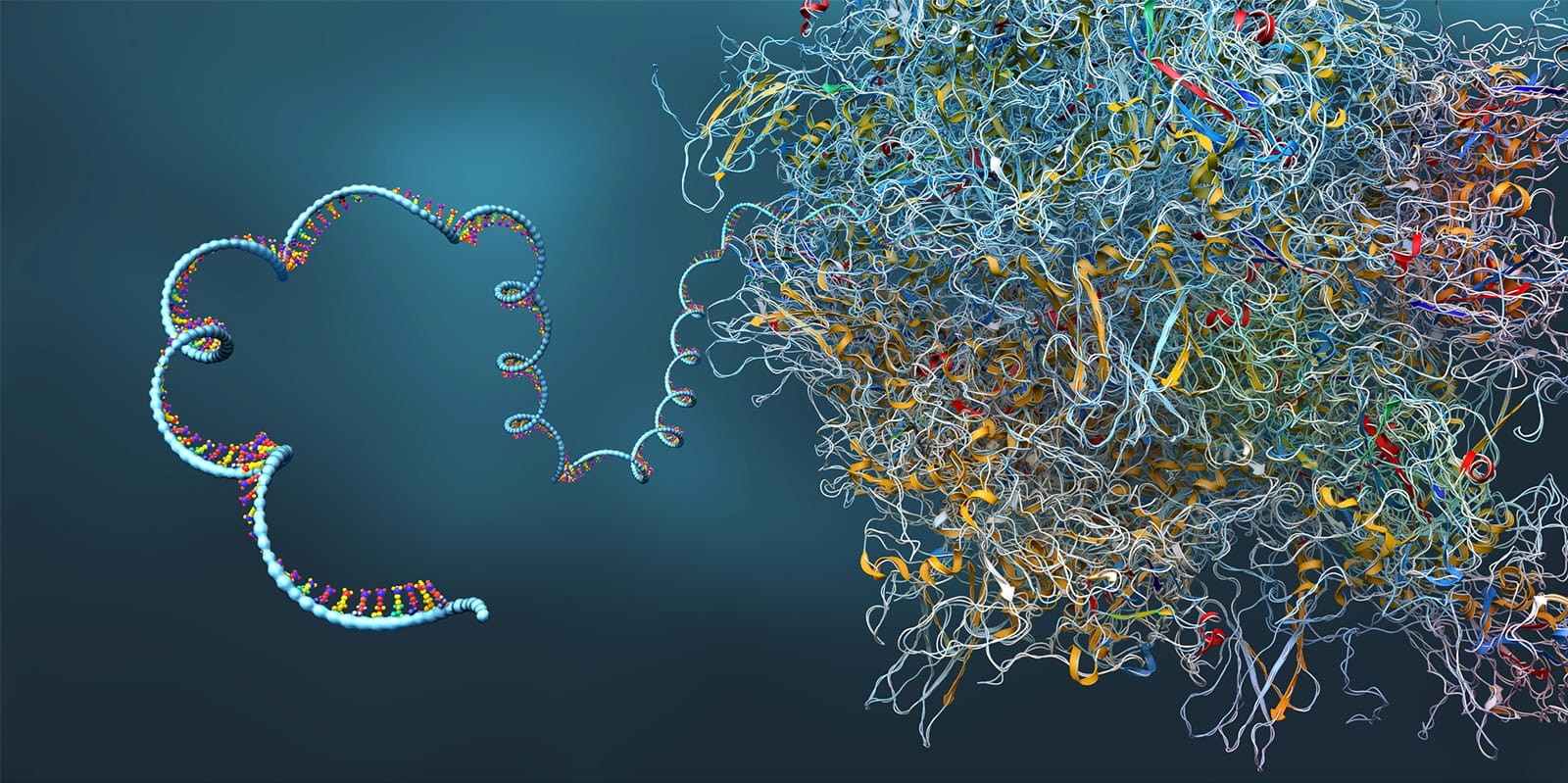
Vallecillo-Viejo, IC, Liscovitch-Brauer, N, Montiel-Gonzalez, MF, Eisenberg, E, Rosenthal, JJC (2018). Abundant off-target edits from site-directed RNA editing can be reduced by nuclear localization of the editing enzyme. RNA Biology. 15:104-114. PMCID: PMC5786015
Buchumenski I, Roth SH, Kopel E, Katsman E, Feiglin A, Levanon EY, Eisenberg E. Global quantification exposes abundant low-level off-target activity by base editors. Genome Res. 2021 Oct 19;31(12):2354–61. PMID: 34667118; PMCID: PMC8647836.
Montiel-Gonzalez MF, Diaz Quiroz JF, Rosenthal JJC. Current strategies for Site-Directed RNA Editing using ADARs. Methods. 2019 Mar 1;156:16-24. PMID: 30502398; PMCID: PMC6814296.
Validation of Nav1.7 as a target for novel analgesics
Dib-Hajj, SD, Rush, AM, Cummins, TR, Hisama, FM, Novella, S, Tyrrell, L, Marshall, L, Waxman, SG (2005) Gain-of-function mutation in Nav1.7 in familial erythromelalgia induces bursting of sensory neurons. Brain, 128:1847-1854. PMID: 15958509
Geha P, Yang Y, Estacion M, Schulman BR, Tokuno H, Apkarian AV, Dib-Hajj SD, Waxman SG (2016). Pharmacotherapy for Pain in a Family With Inherited Erythromelalgia Guided by Genomic Analysis and Functional Profiling. JAMA Neurol. 73(6): 659-67. PMID: 27088781.
Akin EJ, Higerd GP, Mis MA, Tanaka BS, Adi T, Liu S, Dib-Hajj FB, Waxman SG*, Dib-Hajj SD* (2019) Building sensory axons: delivery and distribution of NaV1.7 channels and effects of inflammatory mediators. Sci Adv, 5(10): eaax4755. PMID:3168184. * Co-corresponding authors.
Labau JIR*, Estacion M*, Tanaka BS*, de Greef BTA, Hoeijmakers JGJ, Geerts M, Gerrits MM, Smeets HJM, Faber CG, Merkies ISJ, Lauria G, Dib-Hajj SD†, Waxman SG† (2020) Differential effect of lacosamide on Nav1.7 variants from responsive and non-responsive patients with small fiber neuropathy. Brain, 143(3):771-782. PMID: 32011655 †Co-corresponding authors
Akin EJ, Alsaloum M, Higerd GP, Liu S, Zhao P, Dib-Hajj FB, Waxman SG, Dib-Hajj SD (2021). Paclitaxel increases axonal localization and vesicular trafficking of Nav1.7. Brain. 144(6): 1727-1737. doi: 10.1093/brain/awab113. PMID: 33734317; PMCID: PMC8320304.
Pre-clinical models of pain studies
Burton MD, Tillu DV, Mazhar K, Mejia GL, Asiedu MN, Inyang K, Hughes T, Lian B, Dussor G, Price TJ (2017) Pharmacological activation of AMPK inhibits incision-evoked mechanical hypersensitivity and the development of hyperalgesic priming in mice. Neuroscience. 359 119-129. PMC5641389

Moy, JK, Khoutorsky A, Asiedu MN, Black, BJ, Kuhn JL, Barragan-Iglesias P, Megat S, Burton MD, Burgos-Vega CC, Melemedjian OK, Boitano S, Vagner J, Gkogkas CG, Pancrazio JJ, Mogil JS, Dussor G, Sonenberg N, Price TJ (2017) The MNK-eIF4E signaling axis contributes to injury-induced nociceptive plasticity and the development of chronic pain. Journal of Neuroscience. 37(31) 7481-7499. PMC5546114
Burgos-Vega CC, Quigley LD, Trevisan G, Yan F, Asiedu M, Motina M, Safdar N, Yousuf H, Avona A, Price T, and Dussor G (2018) Non-invasive dural stimulation in mice: a novel preclinical model of migraine. Cephalalgia. 2019 Jan;39(1):123-134. PMC6499065
Jeevakumar V, Al Sardar AK, Mohamed F, Smithhart CM, Price T, Dussor G. (2020) IL-6 induced upregulation of T-type Ca2+ currents and sensitization of DRG nociceptors is attenuated by MNK inhibition. J Neurophysiol. 2020 Jul 1;124(1):274-283. PMC7474456
Yan J, Melemedjian OK, Price TJ, Dussor G. Sensitization of dural afferents underlies migraine-related behavior following meningeal application of interleukin-6 (IL-6). Mol Pain. 2012 Jan 24;8:6. doi: 10.1186/1744-8069-8-6. PMID: 22273495; PMCID: PMC3274468.
Use of human sensory neurons to accelerate translation
North RY, Li Y, Ray P, Rhines LD, Tatsui CE, Rao G, Johansson CA, Zhang H, Kim YH, Zhang B,Dussor G, Kim TH, Price TJ, Dougherty PM (2019) Electrophysiological and transcriptomic correlates of neuropathic pain in human dorsal root ganglion neurons. Brain. 142(5) 1215-1226.
Mis MA, Yang Y, Tanaka BS, Gomis-Perez C, Liu S, Dib-Hajj F, Adi T, Garcia-Milian R, Schulman BR, Dib-Hajj SD, Waxman SG (2019). Resilience to Pain: A Peripheral Component Identified Using Induced Pluripotent Stem Cells and Dynamic Clamp. J Neurosci. 39(3):382-392. PMID: 30459225; PMCID: PMC6335750.
Shiers SI, Klein RM, Price TJ (2020) Quantitative differences in neuronal subpopulations between mouse and human dorsal root ganglia demonstrated with RNAscope in situ hybridization. Pain, 161 (10) 2410-2424.
Middleton SJ, Barry AM, Comini M, Li Y, Ray PR, Shiers S, Themistocleous AC, Uhelski ML, Yang X, Dougherty PM, Price TJ, Bennett DL. Studying human nociceptors: from fundamentals to clinic. Brain. 2021 Jun 22; 144(5): 1312-1335. doi: 10.1093/brain/awab048. PMID: 34128530; PMCID: PMC8219361.
Careers
Research Assistant II/III (RC3-Assay) Rosenthal laboratory
The Rosenthal lab at the MBL has an opening for a full-time high level Research Assistant II/III. Higher ranks are possible depending on the applicant’s experience. The initial period of the hire is for 1 year; however, the position is part of a multi-year project and extensions are possible based on performance. Approximately 50 million Americans suffer from chronic pain, half of which experience it daily. Currently, opiates are our only effective treatment for sever acute and chronic pain and they are causing enormous issues across the country and abroad. For example, more than 2 million Americans are addicted to opiates and close to 50 thousand of them die each year from overdose. There is an urgent need to find alternative, non-addictive treatments. The position is to support research into using RNA editing as a novel non-addictive analgesic for the treatment of pain.
Outreach & Education
NIH HEAL Initiative: The Helping to End Addiction Long-term

The NIH HEAL Initiative is a trans-NIH effort to improve prevention and treatment strategies for opioid misuse and addiction and to enhance pain management. Learn more about the initiative.
Sodium Channels and Pain

RNA Editing